
October 01, 2020
The hype and hope of veterinary cannabis
How should a veterinarian respond when a client asks about treating a pet dog with a cannabinoid tincture advertised as an analgesic? Is there research supporting that use or underlying any of the claims made for the scores of cannabis and cannabinoid-based products marketed for pets? Does a practitioner who recommends such a product for a patient risk running afoul of the state licensing board, the Food and Drug Administration, or the Drug Enforcement Administration?
These were just some of the thorny issues covered during the first-ever AVMA Cannabis Symposium, held Aug. 20-22 during the AVMA Virtual Convention 2020. Speakers addressed various aspects of cannabis as a veterinary therapeutic, such as regulatory and toxicological concerns, as well as its potential as an analgesic or treatment for osteoarthritis in animal patients. Following are some of the speaker highlights.

Genie on the loose
Thirty-three states have legalized marijuana for medicinal or recreational use by people—or both. And yet, none of these laws account for use of cannabis in veterinary medicine. California is the only state to specifically address veterinarians’ ability to engage with clients, indicating that veterinarians can discuss the use of cannabis for medical purposes with clients without being disciplined by the veterinary medical board solely for having that conversation. By that same statute, veterinarians are prohibited from prescribing, dispensing, or administering any cannabis or cannabis-based products. The statute does not address the therapeutic use of products derived from industrial hemp, which are covered under provisions of the state’s veterinary practice act applicable to diagnosing, prescribing, or administering a drug for prevention or treatment of an animal’s condition.
The Food and Drug Administration has approved only one cannabis-derived drug and three synthetic cannabis-related drugs, all for use in human medicine. No other cannabis, cannabis-derived, or cannabidiol product currently available is approved by the agency.
“We certainly recognize the potential opportunities that cannabis-derived compounds may offer and acknowledge the significant interest in these possibilities,” said symposium speaker Randall Gnatt, a senior regulatory counsel in the Office of Surveillance and Compliance in the FDA’s Center for Veterinary Medicine.
“We’re also aware that some companies are marketing products in ways that violate the federal Food, Drug, and Cosmetic Act and then may put the health and safety of people and animals at risk,” he explained. “The agency is committed to protecting the public health while also taking steps to improve the efficiency of regulatory pathways for the lawful marketing of appropriate cannabis-derived products.”
Gnatt said the FDA is conducting a comprehensive evaluation of CBD and related products with a focus on educating the public about these products, informing the agency’s regulatory considerations of these products, and taking action when necessary to protect public health.
“We understand there’s high demand with consumers seeking out these novel products for a variety of perceived health-related or other reasons. But as the agency has stated before, we are concerned that some people wrongly think that the myriad of CBD products on the market have been evaluated by FDA and determined to be safe,” which, as Gnatt explained, isn’t the case.
“Other than the approved human prescription drug, we know little about the potential effects of sustained or cumulative long-term use of CBD,” Gnatt continued. “We don’t know about coadministration with other medicines or risks to vulnerable human and animal populations. This doesn’t mean that we know CBD is categorically unsafe under all circumstances, but given the gaps in our current knowledge and the known risks that have been identified, we’re not at a point where we can conclude that CBD products are safe for use.”
Little is known about the effects of cannabis and CBD on various nonhuman animal species, particularly with regard to the accumulation of residues in the edible tissues of food-producing animals. “There is a great need for more rigorous scientific research into both safety and potential therapeutic uses of cannabis-derived products for animals,” Gnatt said.
Conflicting federal and state laws either prohibiting or sanctioning medical marijuana or hemp-derived CBD can put veterinarians in a difficult spot. “Clients are able to get these products right down the street or through the internet, and they’re looking for advice from their veterinarian,” said Jim Penrod, executive director of the American Association of Veterinary State Boards.
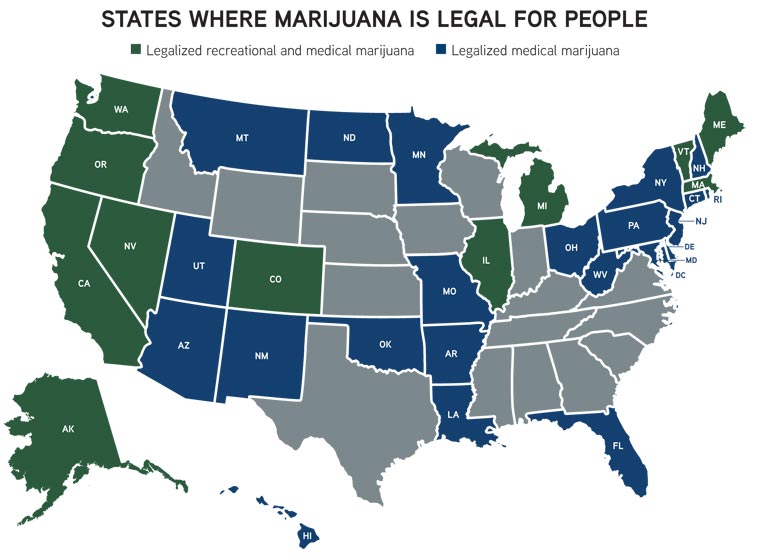
Penrod spoke during the cannabis symposium about the varying views among U.S. veterinary licensing boards about the issue. Marijuana was illegal for decades, he explained, adding that the drug was difficult to study given its classification as a Schedule 1 substance. When California legalized medical marijuana in 2006, the state “let the genie out of the bottle,” as it were, with the decriminalization process quickly outpacing the research.
“Because things are progressing so quickly and decisions are being made so quickly … I’m not going to give you the answers today. I’m not going to tell you that, ‘Yes, it’s fine for you to go talk about cannabis,’ or ‘It’s fine for you to dispense.’ I don’t have those answers,” Penrod said.
In 2019, the AAVSB surveyed state veterinary licensing boards about whether it is legal for a veterinarian to discuss cannabis with a client. Penrod said the association recently contacted those boards to determine whether they were still comfortable with the answers they gave in the 2019 survey, and several changed their answers.
Responses varied from one extreme to the other. Six states said veterinarians could lose their license if they even talk about cannabis, four said veterinarians need to adhere to federal law, seven said state boards can’t even provide legal advice, seven said they have no formal opinion on the matter, two said veterinarians could talk about cannabis but only if the client starts the conversation, 18 responded that veterinarians could discuss cannabis but could not prescribe or dispense it, and four said veterinarians could discuss the topic.
The position of the AAVSB is that veterinarians should be able to discuss CBD with a client to ensure animal and public protection. “That just makes sense,” Penrod said. “If a client comes in and says, ‘I’m going to use CBD on an animal,’ you should be able to talk to them about it, to warn them about some of the side effects, to watch out for those, to make sure that they’re purchasing a product that’s been analyzed and it doesn’t contain things like pesticides.”
The AAVSB has created a task force to create guidance documents for regulatory boards concerning the issue of cannabis. “Because things are changing so quickly, if we drafted regulations or practice law language, it could be out of date as soon as we published it. Guidance is a little more flexible,” Penrod said.
Cannabis in the clinic
Dr. Trina Hazzah is regularly questioned about cannabis use even though there are no cannabis products approved for therapeutic use in animals.
That is, clients frequently ask Dr. Hazzah, a veterinary oncologist working in Los Angeles whose area of interest is complementary and alternative medicine, about incorporating cannabis into their pets’ treatment protocols.
Dr. Hazzah, a founder and the co-president of the Veterinary Cannabis Society, offered her perspective on the therapeutic use of cannabis-derived products as part of the AVMA Cannabis Symposium.
As Dr. Hazzah explained, cannabis is primarily used with animals as an anti-inflammatory, analgesic, anti-anxiety, or anti-neoplastic. Prior to considering a cannabis product, the patient must first be evaluated to confirm that the animal has a potentially cannabis-responsive condition.
“Does the patient have any contraindications or comorbidities that may prevent you from starting cannabis? Are there any potential drug interactions that you should be aware of?” Dr. Hazzah asked. “The next step is to evaluate the actual product as well as the product safety.”
She cited a 2015 study that evaluated 75 edible cannabis products available in various California cities and found that just 7% of the products were accurately labeled for the cannabinoid content. In a follow-up session, Jack Henion, PhD, professor emeritus of toxicology at Cornell University, also conveyed results of a similar study where 12 of 13 animal products had greater THC levels than Canada’s acceptable limits.
“It’s really, really important that clients do their due diligence and ask for a certificate of analysis,” said Dr. Hazzah, who discourages clients from treating pets with cannabis products marketed for human illness.
“You want to walk them through finding companies that are transparent, that have good customer service, that have up-to-date COA—a certificate of analysis—confirming that the product is free of contaminants and that is very specific on what is in the product,” she said.
Talk to clients about potential adverse effects and what signs to look for in pets, Dr. Hazzah added.
“And then, lastly, you should set really clear expectations with a client, making sure that they know that cannabis is not necessarily a wonder drug,” Dr. Hazzah explained.
Wrapping up
Dr. Dharati Szymanski, an assistant director in the AVMA Division of Animal and Public Health and organizer of the summit, summarized the event thus: “Our members hear varying perspectives from cannabis manufacturers, their state boards, regulatory agencies, colleagues, and, of course, clients. Sometimes it is difficult to see where these perspectives might intersect or how far apart they sit. Practitioners want to have confidence in the safety and efficacy of products. However, when the marketplace has outpaced the evaluation of products, veterinarians need to understand the potential benefits as well as risks surrounding these products for their patients and the liability risks for themselves. There has been much progress in bridging these gaps, but we need more work in areas of research, quality control, and FDA evaluation for veterinarians to have general confidence in available products.”